Gene Therapies
Pioneering the future of medicine
With a track record of success across multiple gene therapy modalities, we combine scientific excellence, deep regulatory insights and operational agility to accelerate your program from concept to approval, ensuring your therapy reaches the patients who need it most.
Unmatched gene therapy expertise
Count on our experience in gene therapy, with deep knowledge across multiple modalities, including viral vectors, gene editing and gene silencing.
Agility in complex trials
Navigate the complexities of gene therapy clinical trials with an adaptive partner, delivering speed, precision and regulatory compliance at every phase.
End-to-end support
From early-stage development to post-market studies, our integrated approach provides superior support, boosting your odds of success and patient access.
Global insights for informed go/no-go decisions
Wherever your trial takes you, our global presence and extensive expertise help deliver the data you need for informed go/no-go decisions. We’ve advanced gene therapy trials across:
- North America: Canada, U.S.
- South America: Brazil, Peru
- Europe: Austria, Belgium, Czechia, Denmark, Finland, France, Germany, Greece, Israel, Italy, Netherlands, Poland, Russia, Spain, Sweden, Switzerland, Turkey, United Kingdom
- Africa: South Africa
- Asia-Pacific: Australia, China, India, Japan, South Korea, Taiwan
Gene therapy experience that matters
In the last 5 years, we have supported:
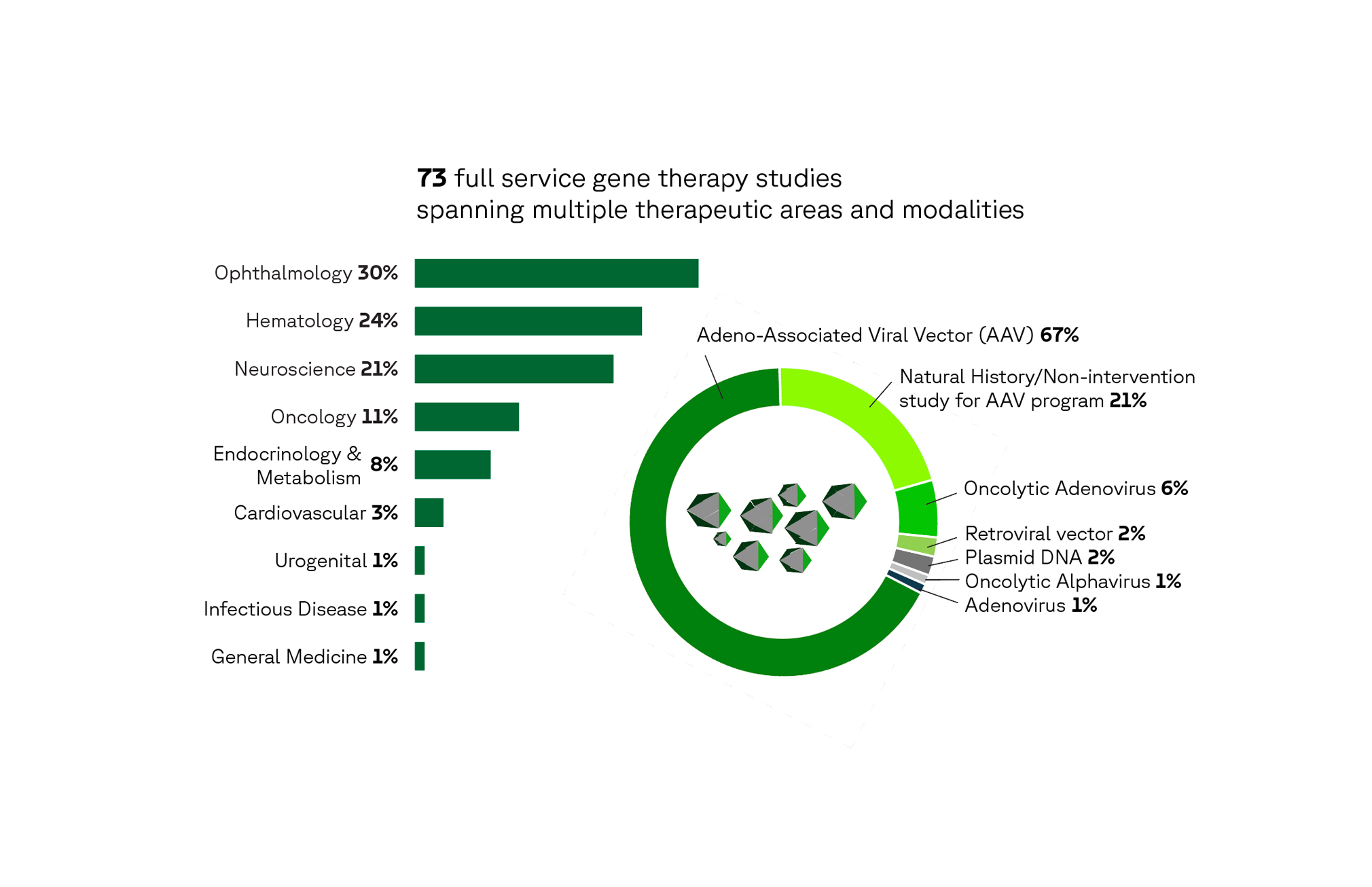
Our experience in gene therapy
In the last five years, our gene therapy experience spans ophthalmology (30%), hematology (24%), neuroscience (22%), oncology (11%), endocrinology/metabolism (6%) and other areas, including cardiovascular, urogenital, infectious disease and general medicine.
Modalities have included AAV, retroviral vector, plasmid DNA and both viral and non-viral delivery systems (e.g., adenovirus, LNP), as well as gene editing technologies like CRISPR and meganucleases. Our work also includes natural history and non-intervention studies.
Seamless delivery for your next trial
Working as an extension of your team, our multidisciplinary approach ensures comprehensive support to navigate the complexities of your gene therapy program with precision and expertise.
-
Scientific and medical expertise
We don’t just understand the complexities of cell and gene therapies; we know how they work at a scientific level. Our expertise extends to advanced therapeutics like RNA-based therapies (e.g., ASOs, siRNAs), T cell engagers and genetically modified organisms.
We support your trial by addressing the unique challenges posed by these treatments:
- Protocol development
- Long-term follow-up (LTFU)
- Development of companion diagnostics
- GMO and ATMP global regulatory requirements
- Vendor qualification
- Medical monitoring
-
Global operational solutions
-
Customized training
Related indications
The clock is ticking. Tap into our multidisciplinary experts to get the most from your cell therapy study. Together, we’ll improve your odds of success and address urgent, unmet medical needs.
Explore our related areas of expertise.

