Promoting access to innovative cardiac medical devices through diversity and inclusion
Promoting access to innovative cardiac medical devices through diversity and inclusion
Jill Eddy, MS, Associate Director, Strategic Delivery and Growth
Dr. Lisa Moore, Executive Director, Medical Device and Diagnostics Development
Mauricio Scarcello, MBA, Director, Operational Strategy and Planning, Medical Device and Diagnostics Development
Minimally invasive cardiovascular devices can provide life-saving treatment and reduce the need for open-heart procedures, but patient access to these devices is not equal. Recognizing the need to improve delivery and access to cardiovascular technologies, our team at Fortrea is sharing our experience with reaching diverse patient populations in clinical trials to improve healthcare access for all.
Inequities in cardiovascular device access
Consider the example of transcatheter aortic valve replacement (TAVR), which uses a bioprosthetic valve to treat patients with severe aortic stenosis. Use of this high-tech medical device has rapidly increased over the past 10 years to become the standard of care.[1] However, studies have noted access to TAVR is lower in geographic areas with higher proportions of patients of Black race and Hispanic ethnicity due to “structural racial, ethnic, and socioeconomic barriers to high-quality care.”[2]
Beyond TAVR, disparities in patient access to novel cardiac medical devices can also be attributed to:
- So-called “healthcare deserts” with a lack of skilled care providers
- Delays in physician experience with new products
- Delays in national coverage determinations, which set criteria and volume requirements to start programs
Access can also be improved at the product development phase. The FDA has issued guidance on Diversity Plans[3] for our industry to better represent underrepresented racial and ethnic populations into clinical trials. Beyond these populations, the FDA also encourages the inclusion of other underrepresented populations, such as people living in rural communities without access to care as well as women who may be underserved and underrepresented in certain indications.
Reflecting diversity in a trial by identifying the right sites
At Fortrea, we have extensive experience identifying the right sites to support diversity within clinical trials. With access to data from thousands of studies—and collaboration from medical, regulatory, clinical teams and our site partners—we can identify the right sites to identify the right populations for a study and speed recruitment.
To augment efficacious study design, we employ digital and healthcare innovations. For example, eConsent, telemedicine, and Mobile Clinical Services help reach patients that may not otherwise be able to easily travel to the clinic. These technologies and services can facilitate blood draws, nurse visits and support certain study procedures in the comfort of a patient’s home.
Along with other digital and mobile capabilities, these patient-centric solutions can:
- Decrease the burdens placed on sites, patients and caregivers
- Support recruitment and retention
- Increase diversity while maintaining quality data and endpoint protection
Site selection can also be enhanced by using U.S. Census data and Fortrea’s investigator performance data. By focusing our attention to diverse demographic areas, our team at Fortrea can target not only internally experienced sites and site partners but also draft carefully crafted feasibility survey questions. This allows us to find new sites in these targeted areas and reach the right patient populations for a study.
Figure 1. Site Selection Considerations for Diversity in the U.S. Sites and Census Data
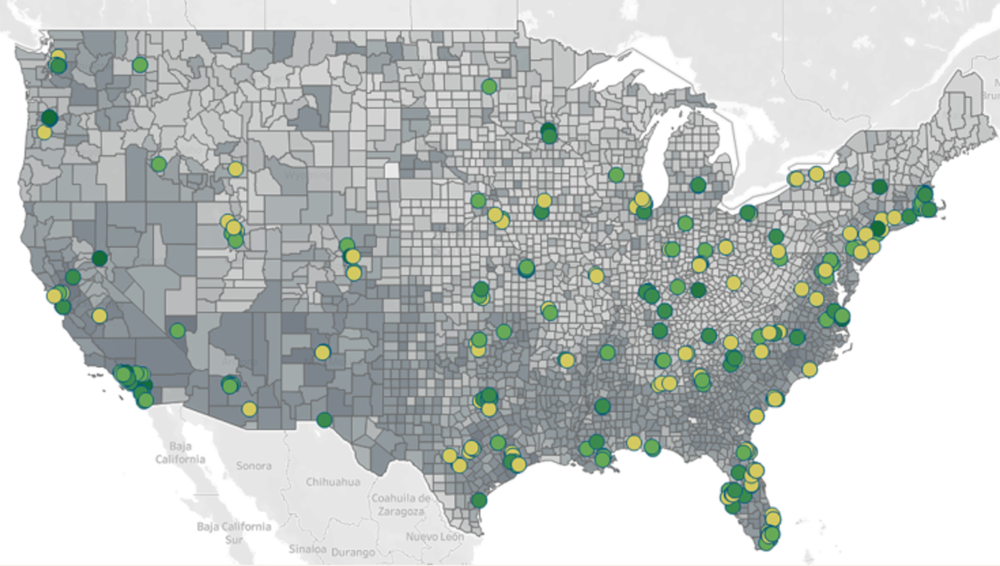
Above: By combining U.S. Census data (2020), real-world data from Labcorp*, and investigator performance scores, Fortrea can identify study sites with excellence performance in or near geographic areas inhabited by diverse patient populations to help boost racial and ethnic diversity in patient recruitment.
*Fortrea will maintain exclusive CRO access to the Labcorp data that supports its clinical-enabling solutions for a fixed period of time.
By combining the right sites with the right strategy and technologies, we can meet our sponsors’ goals and satisfy performance metrics as outlined by the FDA requirements for a Diversity Plan.
Please visit our Cardiovascular Disease and Medical Device and Diagnostic Solutions pages to learn more about how we can help you find the right sites and patient populations to advance your cardiology and medical device trials.
[1] Carroll J, Mack M, Vemulapalli S, et al. STS-ACC TVT Registry of Transcatheter Aortic Valve Replacement. J Am Coll Cardiol. 2020 Nov, 76 (21) 2492–2516.
[2] Nathan AS, Yang L, Yang N, et al. Racial, Ethnic, and Socioeconomic Disparities in Access to Transcatheter Aortic Valve Replacement Within Major Metropolitan Areas. JAMA Cardiol. 2022;7(2):150–157. doi:10.1001/jamacardio.2021.4641
[3] U.S. Food and Drug Administration. Diversity plans to improve enrollment of participants from underrepresented racial and ethnic populations in clinical trials; draft guidance for industry 2022. https://www.fda.gov/media/157635/download. Accessed October 6, 2023
